QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Four options are provided as possible answers to the following questions. Choose the
answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in
the ANSWER BOOK, e.g. 1.11 E.
1.1 Which ONE of the following compounds has the HIGHEST vapour pressure?
A HCOOH
B CH3CHO
C CH3CH2OH
D CH3CH2CH3 (2)
1.2 Which ONE of the formulae below represents the product of a
POLYMERISATION reaction?

1.3 Which ONE of the following combinations are BOTH UNSATURATED
HYDROCARBONS?
A Ethane and ethene
B Ethene and ethyne
C Ethane and ethanol
D Ethanoic acid and ethene (2)
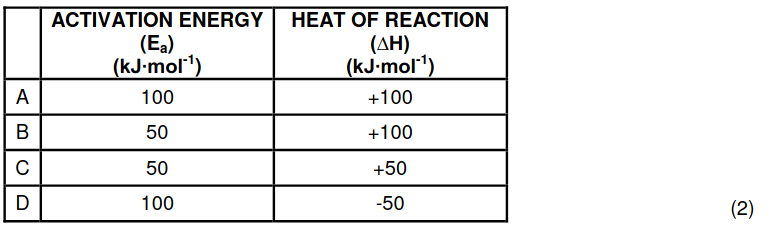
1.4 Which ONE of the following sets of values for activation energy (Ea) and heat
of reaction (∆H) is possible for a reaction?
1.5 Consider the following balanced equation for a system at equilibrium:
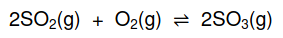
How will the addition of a catalyst to the equilibrium mixture affect the YIELD
and REACTION RATE?

1.6 A hypothetical reaction reaches equilibrium at a certain temperature in a
closed container according to the following balanced equation:
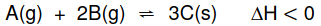
Which ONE of the following changes to the equilibrium conditions will result in
an INCREASE in the equilibrium constant, Kc?
A Increase in temperature
B Decrease in temperature
C Increase in pressure at constant temperature
D Decrease in pressure at constant temperature (2)
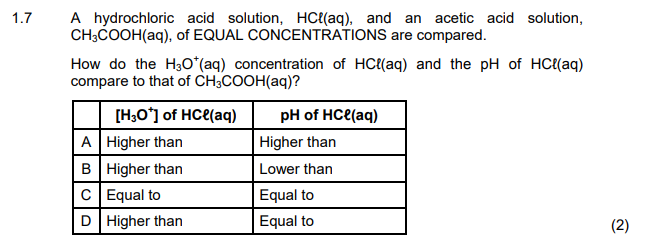
1.8 Two hypothetical half-reactions and their respective reduction potentials are
shown below:

A galvanic cell is set up using the above substances.
Which ONE of the following statements is CORRECT for this galvanic cell?
A B(s) is the reducing agent.
B A(s) is the oxidising agent.
C The mass of B(s) will increase.
D The mass of A(s) will decrease. (2)
1.9 In an electrolytic cell …
A the anode is the positive electrode.
B oxidation takes place at the cathode.
C electrons flow from the cathode to the anode.
D the mass of the anode increases. (2)
1.10 Which ONE of the following is used as a catalyst in the Ostwald process?
A Iron
B Copper
C Platinum
D Vanadium (V) oxide (2)
[20]
