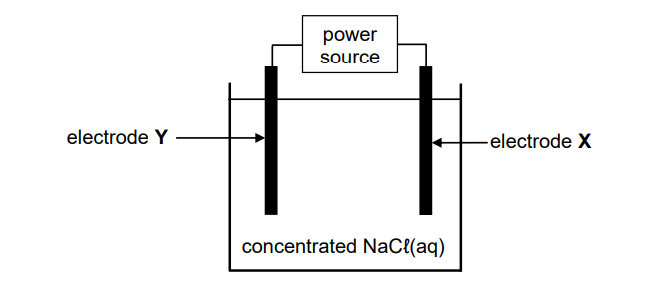
The simplified diagram below represents an electrochemical cell used for the electrolysis of a concentrated sodium chloride solution, NaCℓ(aq). X and Y are carbon electrodes.
9.1 Define the term electrolysis. (2)
9.2 Chlorine gas, Cℓ2(g), is released at electrode X.
Write down the:
9.2.1 Letter (X or Y) of the electrode where oxidation takes place (1)
9.2.2 Half-reaction that takes place at electrode Y (2)
9.2.3 Direction in which electrons flow in the external circuit
Choose from X to Y OR Y to X. (1)
9.2.4 Balanced equation for the net (overall) cell reaction that takes
place in the cell (3)
9.3 How will the pH of the electrolyte change during the reaction?
Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
9.4 Give a reason for the answer to QUESTION 9.3. (1)
[11]
TOTAL: 150
