Hydrogen gas, H2(g), reacts with sulphur powder, S(s), according to the following
balanced equation:
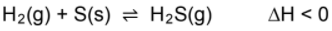
The system reaches equilibrium at 90 °C.
6.1 Define the term chemical equilibrium. (2)
6.2 How will EACH of the following changes affect the number of moles of H2S(g) at equilibrium?
Choose from INCREASES, DECREASES or REMAINS THE SAME.
6.2.1 The addition of more sulphur (1)
6.2.2 An increase in temperature
Use Le Chatelier’s principle to explain the answer. (4)
6.3 The sketch graph below was obtained for the equilibrium mixture.
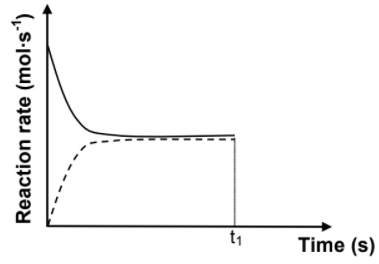
A catalyst is added to the equilibrium mixture at time t1. Redraw the graph above in your ANSWER BOOK. On the same set of axes, complete the graph showing the effect of the catalyst on the reaction rates. (2)
Initially 0,16 mol H2(g) and excess S(s) are sealed in a 2 dm3 container and the system is allowed to reach equilibrium at 90 °C.
An exact amount of Pb(NO3)2 solution is now added to the container so that ALL the H2S(g) present in the container at EQUILIBRIUM is converted to PbS(s) according to the following balanced equation:
Pb(NO3)2(aq) + H2S(g) → PbS(s) + 2HNO3(aq)
The mass of the PbS precipitate is 2,39 g.
6.4 Calculate the equilibrium constant Kc for the reaction H2(g) + S(s) ⇌ H2S(g) at 90 °C. (9)
[18]
