8.1 Learners set up a galvanic cell and measure its emf under standard conditions.
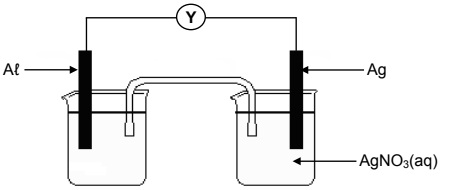
8.1.1 Write down the name of component Y. (1)
8.1.2 Is Al the ANODE or the CATHODE? (1)
8.1.3 Write down the overall (net) cell reaction that takes place in this cell when it is working. (3)
8.1.4 Calculate the initial emf of this cell. (4)
8.2 Consider the half-cells, P, Q and R, represented in the table below.
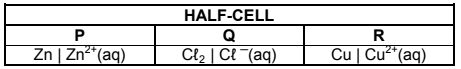
Different combinations of the half-cells above are compared to determine the highest emf produced under standard conditions.
8.2.1 Write down the NAME of a suitable electrode for half-cell Q. (1)
8.2.2 State the standard conditions under which the half-cells should operate to ensure a fair comparison. (2)
8.2.3 Write down the NAME or FORMULA of the strongest reducing agent in the half-cells above. (1)
8.2.4 Which combination of half-cells will produce the highest emf?
Choose from PR, PQ or QR. (NO calculation is required.) (1)
[14]
