7.1 Ammonia ionises in water to form a basic solution according to the following balanced equation:
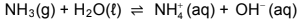
7.1.1 Is ammonia a WEAK or a STRONG base? Give a reason for the answer. (2)
7.1.2 Write down the conjugate acid of NH3(g). (1)
7.1.3 Identify ONE substance in this reaction that can behave as an ampholyte in some reactions. (1)
7.2 A learner adds distilled water to a soil sample and then filters the mixture. The pH of the filtered liquid is then measured. He then gradually adds an ammonia solution, NH3(aq), to this liquid and measures the pH of the solution at regular intervals. The graph below shows the results obtained.

7.2.1 Is the soil sample ACIDIC or BASIC? Refer to the graph above and give a reason for the answer. (2)
7.2.2 Calculate the concentration of the hydroxide ions ( − OH ) in the reaction mixture after the addition of 4 cm3 of NH3(aq). (4)
7.3 A laboratory technician wants to determine the concentration of a hydrochloric acid (HCl) sample. He adds 5 cm3 of the HCl sample to 495 cm3 of distilled water to give 500 cm3 of dilute hydrochloric acid, HCl(aq).
During a reaction 50 cm3 of this dilute hydrochloric acid solution, HCl(aq), reacts completely with 0,29 g of sodium carbonate, Na2CO3(s).

The balanced equation for the reaction is:
Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + CO2(g) + H2O(l)
Calculate the concentration of the hydrochloric acid sample. (7)
[17]
