1.1 The IUPAC name of an organic compound with molecular formula C7H14O2:
A Heptanal
B Heptan-1-ol
C Heptan-2-ol
D Heptanoic acid (2)
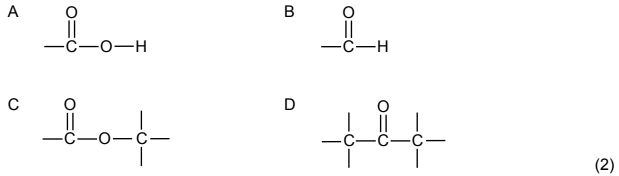
1.2 Which ONE of the following structures is the functional group of aldehydes?
1.3 Which ONE of the following equations represents a cracking process?
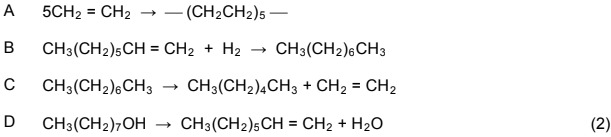
1.4 The potential energy diagram for a chemical reaction is shown below.
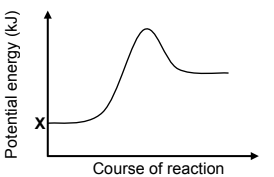
I: X represents the potential energy of the products formed during the reverse reaction.
II: The graph could be a representation of the change in potential energy for the following reaction:
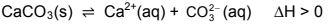
III: The graph could be a representation of the change in potential energy for the combustion of methane.
Which of the statements above are TRUE?
A I and II only
B II and III only
C I and III only
D I, II and III (2)
1.5 A certain chemical reaction reaches equilibrium at 25 °C. The equilibrium constant, Kc, for the reaction at this temperature is 1,0 x 10-4.
Which ONE of the following statements regarding this reaction at equilibrium
is CORRECT?
A The concentration of the products is equal to that of the reactants.
B The concentration of the products is higher than that of the reactants.
C The concentration of the products is lower than that of the reactants.
D The rate of the forward reaction is lower than the rate of the reverse reaction. (2)
1.6 Consider the following chemical reaction at equilibrium in a closed container:
2HgO(s) ⇌ 2Hg(l) + O2(g)
More HgO(s) is now added to the container at constant temperature. How will the number (in moles) of O2(g) and the value of Kc be affected at equilibrium?
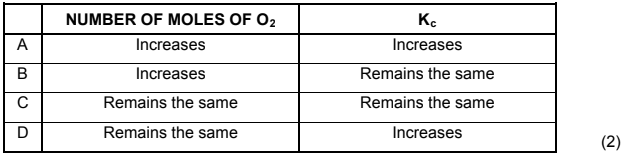
1.7 Which ONE of the following solutions, each of concentration 0,1 mol∙dm-3, has the highest pH?
A HNO3(aq)
B NH4Cl(aq)
C Na2CO3(aq)
D CH3COOH(aq) (2)
1.8 The cell notation for a galvanic cell is as follows:
Ni(s) | Ni2+ (1 mol∙dm-3) || Pb2+ (1 mol∙dm-3) | Pb(s)
Which ONE of the following statements is CORRECT for this cell?
A Ni is oxidised.
B Pb(s) is reduced.
C Ni2+(aq) is the oxidising agent.
D Pb2+ is the reducing agent. (2)
1.9 Which ONE of the following combinations CORRECTLY shows the products formed during the electrolysis of a CONCENTRATED sodium chloride solution?
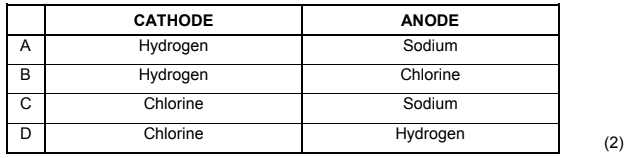
1.10 Which ONE of the following is NOT part of the eutrophication process?
A Algal bloom
B Bacterial nitrogen fixation
C Depletion of oxygen in water
D Increase in plant nutrients in water (2)
[20]
