Learners investigated the relationship between the concentration of a silver nitrate (AgNO3) solution and its conductivity at a constant temperature.
They set up the apparatus, as shown below, and recorded the current. The initial reading of the ammeter was taken before anhydrous AgNO3 was added to distilled water.
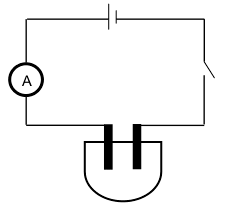
The anhydrous AgNO3 was added to 200 cm3 distilled water spoon by spoon.
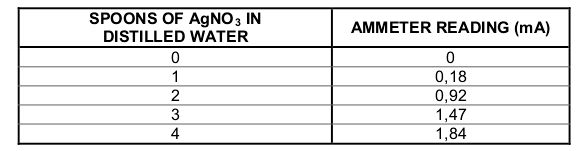
The ammeter reading was recorded after each spoon was added. The results are
shown in the table below.
7.1 Give a reason why the ammeter reading was initially zero. (1)
7.2 Give ONE word/term for a solution that conducts electricity. (2)
7.3 Write down a balanced chemical equation to show how AgNO3 dissociates in water. (2)
7.4 Write down the following for the investigation above:
7.4.1 A hypothesis (2)
7.4.2 Dependent variable (1)
7.4.3 Independent variable (1)
7.4.4 Controlled variable (1)
7.5 Define the term anhydrous. (1)
7.6 If the mass of AgNO3 is 5,3 g per spoon, calculate the concentration of the
solution after TWO spoons have been added. (4)
7.7 Can tap water be used for this experiment? Give a reason for the answer. (2)
7.8 From the results, deduce the relationship between the ion concentration in the
solution and its conductivity. (2)
7.9 A learner accidentally dropped hydrochloric acid into the solution.
7.9.1 How will this affect the ammeter reading? Write down only
INCREASE, DECREASE or REMAIN THE SAME. (1)
7.9.2 Explain the answer to QUESTION 7.9.1. (2)
[22]
