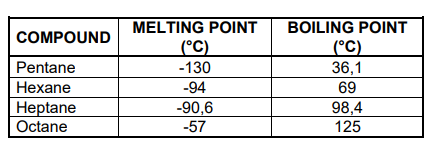
The melting points and boiling points of four straight-chain ALKANES are shown in the table below.
3.1 Define the term melting point. (2)
3.2 Write down the general conclusion that can be made about the melting points of straight-chain alkanes. (2)
3.3 Name the type of Van der Waals forces between molecules of octane. (1)
3.4 Write down the predominant phase of the following alkanes at -100 °C.
Choose from GAS, LIQUID or SOLID.
3.4.1 Pentane (1)
3.4.2 Octane (1)
3.5 Hexane is now compared to 2,2-dimethylbutane.
3.5.1 Is the molecular mass of hexane GREATER THAN, LESS THAN or EQUAL to that of 2,2-dimethylbutane?
Give a reason for the answer. (2)
3.5.2 Is the boiling point of 2,2-dimethylbutane HIGHER THAN, LOWER THAN or EQUAL TO that of hexane? (1)
3.5.3 Fully explain the answer to QUESTION 3.5.2. (3)
[13]
