1.1 Which formula shows the way in which atoms are bonded in a molecule but
does not show all the bond lines?
A. Empirical
B. Molecular
C. Structural
D. Condensed structural (2)
1.2 Which ONE of the following compounds has hydrogen bonds between its
molecules?
A. CH3(CH2)2CH3
B. CH3COCH2CH3
C. CH3COOCH2CH3
D. CH3CH(OH)CH2CH3 (2)
1.3 Consider the compound below.
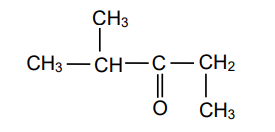
Which ONE of the following is the IUPAC name of this compound?
A. 2-methylpentan-3-one
B. 4-methylpentan-3-one
C. 2,3-dimethylbutan-2-one
C. 2,2,4-trimethylpropan-2-one (2)
1.5 The Maxwell-Boltzmann distribution curve P represents the number of molecules against kinetic energy for a certain reaction.
Curve Q is obtained after a change was made to one reaction condition.
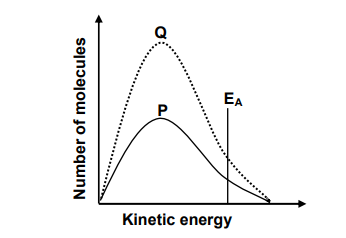
Which ONE of the following changes resulted in curve Q?
A. Addition of a catalyst
B. Increase in temperature
C. Increase in activation energy
D. Increase in the concentration of the reactants (2)
1.6 The expression for the equilibrium constant (Kc) of a hypothetical reaction is given as follows:
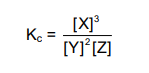
Which ONE of the following equations for a reaction at equilibrium matches the above expression?
A. Z(g) + 2Y(g) ⇌ 3X(s)
B. Z(aq) + 2Y(aq) ⇌ 3X(ℓ)
C. Z(g) + Y2(g) ⇌ 3X(aq) + Q(s)
D. Z(aq) + 2Y(aq) ⇌ 3X(aq) + Q(s) (2)
1.7 Two dilute acids of equal concentrations are added to separate test tubes as shown below.
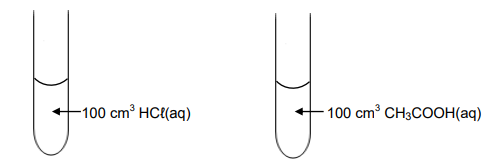
Consider the following statements regarding these acids:
I: The pH of each is less than 7.
II: Both will react at the same rate with 5 g of magnesium powder.
III: Both will neutralise the same number of moles of NaOH(aq).
Which of the statements above is/are TRUE?
A. I only
B. I, II and III
C. I and III only
D. II and III only (2)

1.9 The diagram below represents a voltaic cell.
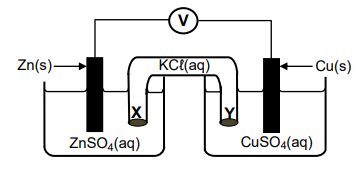
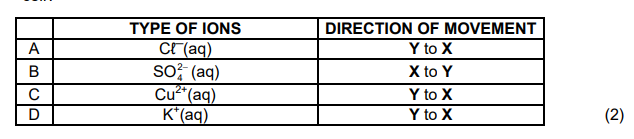
Which ONE of the following correctly describes the movement of ions in the cell?
Which ONE of the following statements is TRUE?
A. X is made of platinum.
B. The mass of X increases.
C. X is the electrode where oxidation takes place.
D. X is connected to the positive terminal of the power supply. (2)
[20]
