1.1 Which ONE of the following compounds has hydrogen bonds between
molecules?
A. Pentanal
B. Pentan-2-one
C. Pentanoic acid
D. Methyl butanoate (2)
1.2 To which homologous series does a compound with molecular formula
C6H12O2 belong?
A. Ketones
B. Alcohols
C. Aldehydes
D. Carboxylic acids (2)
1.3 Which functional groups are involved in the formation of esters?
A. Formyl and carbonyl
B. Hydroxyl and carbonyl
C. Hydroxyl and carboxyl
D. Carbonyl and carboxyl (2)
1.4 The equation below represents a reaction at equilibrium.
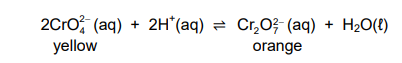
Which ONE of the following will change the colour of the mixture from yellow
to orange?
A. Addition of sodium hydroxide pellets
B. Addition of concentrated hydrochloric acid
C. Increase in pressure at constant temperature
D. Decrease in pressure at constant temperature (2)
1.5 Consider the potential energy graph for the reaction shown below.
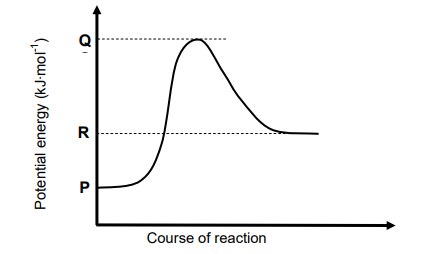
The activation energy for the FORWARD reaction in terms of P, Q and R is:
A. Q
B. R − P
C. Q − R
D. Q − P (2)
1.6 A reaction reaches equilibrium in a closed container according to the following balanced equation:
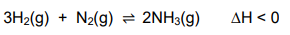
1.7 Sulphuric acid ionises in water according to the following equations:
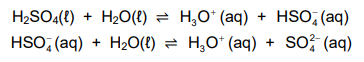
Consider the following statements regarding the ionisation above:

Which of the statements above is/are TRUE?
A. I only
B. I and II
C. I and III
D. I, II and III (2)
1.8 Which ONE of the following reactions, when used in a voltaic cell, will give a positive reading on the voltmeter?
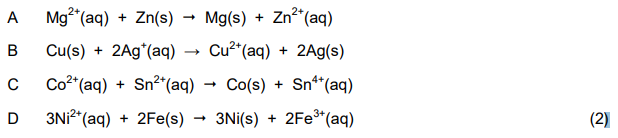
1.9 Which ONE of the following statements is CORRECT for an ELECTROLYTIC
CELL?
A. The anode is the positive electrode.
B. The cathode is the positive electrode.
C. Oxidation takes place at the cathode.
D. Reduction takes place at the anode. (2)