1.1 Which ONE of the following compounds has the LOWEST melting point?
A. Hexane
B. Ethane
C. Butane
D. Octane (2)
1.2 When CH2 = CH2 is converted to CH3CH3, the type of reaction is …
A. hydration.
B. hydrolysis.
C. halogenation.
D. hydrogenation. (2)
1.3 Which ONE of the following compounds in solution will change the colour of
bromothymol blue?
A. CH3CH2CHO
B. CH3CH2COOH
C. CH3CH2COCH3
D. CH3CH2COOCH3 (2)
1.4 Two DIFFERENT samples of IMPURE CaCO3 of EQUAL masses react with 0,1 mol∙dm-3 H2SO4. Assume that the impurities do not react.
The graph below shows the volume of CO2(g) produced for each reaction.
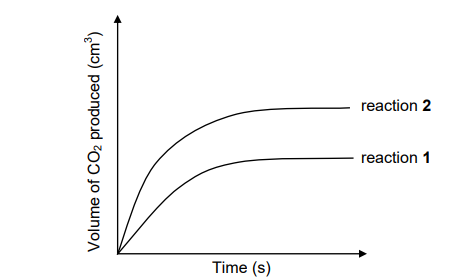
When compared to reaction 2, which ONE of the following statements BEST
explains the curve obtained for reaction 1?
A. The temperature is higher in reaction 1.
B. The surface area is greater in reaction 2.
C. The amount of impurities is greater in reaction 2.
D. The amount of impurities is greater in reaction 1. (2)
1.5 The equation below represents a hypothetical reaction.
A(g) + B(g) ⇌ C(g) ΔH = – 50 kJ·mol-1
The activation energy for the REVERSE reaction is 110 kJ·mol-1 .
Which ONE of the following is the activation energy (in kJ·mol-1) for the FORWARD reaction?
A. 50
B. 60
C. 110
D. 160 (2)
1.6 A reaction reaches equilibrium at 25 °C in a flask according to the following balanced equation:
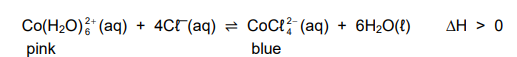
Which ONE of the following will change the colour of the mixture from pink to blue?
A. Adding water
B. Cooling the flask
C. Adding NaOH(aq)
D. Adding NH4Cℓ(aq) (2)
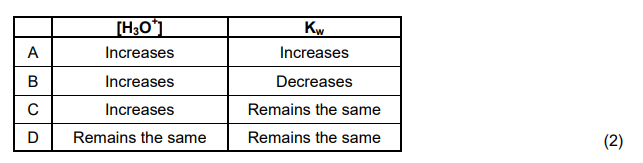
1.7 Dilute nitric acid is added to distilled water at 25 °C. How will this affect the hydronium ion concentration [H3O+] and the ionisation constant (Kw) of water at 25 °C?
1.8 Consider the ionisation reactions I and II
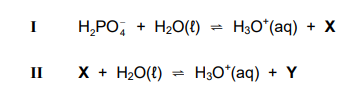
Which ONE of the following combinations represents the formulae of X and Y respectively?

1.9 An electrochemical cell was set up using a Hg(ℓ)|Hg2+(aq) half-cell and
another half-cell under standard conditions.
Which ONE of the following half-cells, when connected to the Hg(ℓ)|Hg2+(aq)
half-cell, will result in the HIGHEST cell potential?
A. Aℓ(s)|Aℓ3+(aq)
B. Zn(s)|Zn2+(aq)
C. Co(s)|Co2+(aq)
D. Pt(s)|H2(g)|H+(aq) (2)
1.10 The following reaction takes place in an electrochemical cell:
CuCℓ2(aq) → Cu(s) + Cℓ2(g)
Which ONE of the following is CORRECT for this cell?
A. It is a galvanic cell.
B. A power source is needed.
C. The reaction is spontaneous.
D. Copper acts as the oxidising agent. (2)
[20]
